Packaging glass bottles safely and efficiently in cartons

Thanks to the new Uhlmann C 2155 cartoner, Laves can reliably pack its medicines and food supplements in cartons.
Modernization of the production line in Schötz (Switzerland)
Laves-Arzneimittel GmbH, a family-owned company with over 100 years of experience, is a medium-sized, German-Swiss pharmaceutical company with two sites, in Hanover (Germany) and Schötz (Switzerland). The approximately 50 employees develop and produce medicinal products and food supplements to support the intestines and strengthen the immune system. The microbiological products are mainly produced using biotechnological processes.
Since 2015, the site in Schötz has been gradually modernized and brought up to the latest state of pharmaceutical technology. The modernization covered the entire production value chain and also had to meet the requirements regulated by the authorities.
In detail, this meant:
- Restructuring of the material flow
- Adaptation of the hygiene zones with airlock systems and clean rooms
- Process-controlled production lines for fermentation and formulation
- Picking with labeling and packaging system
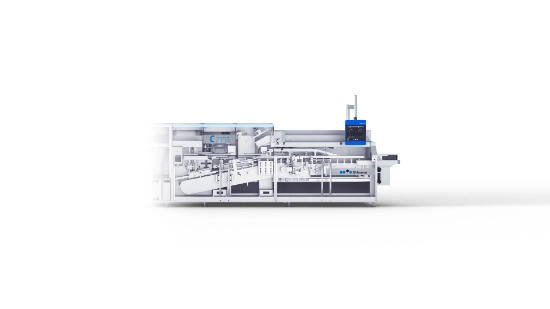
The new C 2155 cartoner from Uhlmann
A packaging system that was over 30 years old, a lack of spare parts, costly maintenance work and increasing quality requirements were the deciding factors for investing in a modern and efficient packaging system.
Since January 2025, the Uhlmann C 2155 cartoner has been handling the packaging of Laves’ products, which are sensitive in two respects – they contain temperature-sensitive ingredients and are packaged in fragile glass bottles.
„I am very satisfied with the cooperation and performance of Uhlmann’s employees: The speedy and reliable installation of the cartoning machine, which only required a brief and pre-calculated production stop, was very impressive – not to mention the aesthetics of the industrial work of art.‟

Dr. Viktor Helbling
Site Manager, Laves-Arzneimittel GmbH, Switzerland


Short channels, reliable solutions and rapid achievement of your goal
Reliability and trust are particularly important for medium-sized companies like Laves: The entire project flow – from the initial contact to the commissioning of the machine – was characterized by partnership-based cooperation and short communication channels.
The positive atmosphere in the project team – during reference visits, at the Factory Acceptance Test (FAT) in Laupheim or during assembly in Schötz – was always noticeable and guaranteed the smooth and efficient progress of the project and the excellent project result.
„Personally, I was impressed by the open, fair and cooperative way in which everyone worked together. We were on an equal footing in the dialog and pulled together from the start – which is why we were able to reach our goal quickly.‟

Axel Friese
Sales Manager at Uhlmann Switzerland
A future-proof investment – the C 2155 in use at Laves
Particular focus was placed on only interrupting production briefly for the changeover. Despite the short time window and limited space, the Uhlmann team managed to complete the installation within the defined time frame – proof of strong logistics and efficient project management.
Our task was to install the system in such a way that two different bottle formats could be reliably and securely packed together with packaging information in cartons:
- 50 ml glass bottle including measuring cup
- 100 ml glass bottle including measuring cup
The investment in the packaging system from Uhlmann is paying off for Laves: Production interruptions are minimized, quality standards are ensured and the conditions for further growth are created. This is because the Uhlmann solution not only offers efficiency, but is also future-proof: The machine allows scaling up to 150 bottles/minute and an extension to 200 ml bottles would also be possible.
Impressions from the Laves-Uhlmann project
Three questions for Dr. Viktor Helbling
Site Manager, Laves-Arzneimittel GmbH, Switzerland
About Laves-Arzneimittel
The company site in Schötz was founded in 1998. Today, around two dozen people work in Schötz. These people are employed in the areas of research and development, quality control, quality assurance, production and administration. The microbiological products are produced using biotechnological processes at the site in Switzerland. Schötz is also home to the research and development department, which focuses on the immune system and various intestinal diseases.